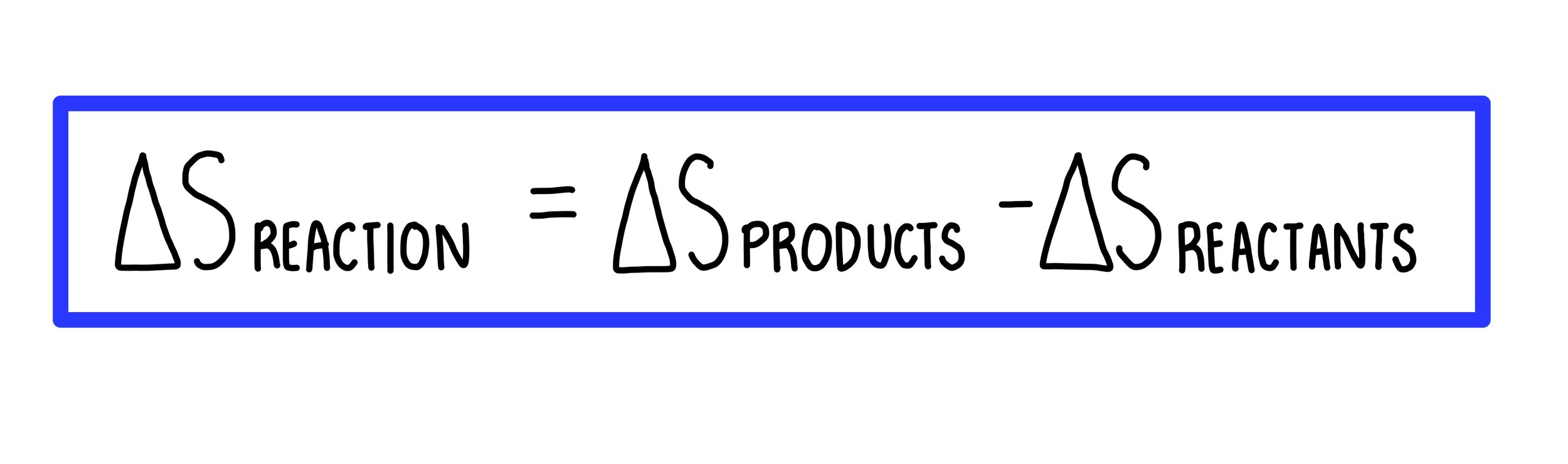
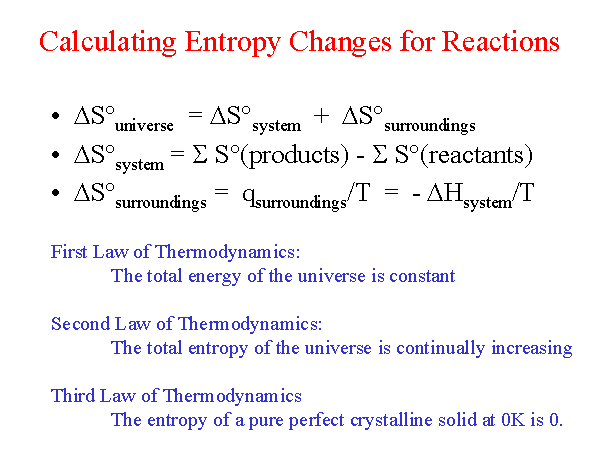
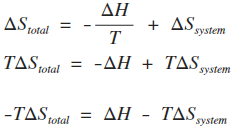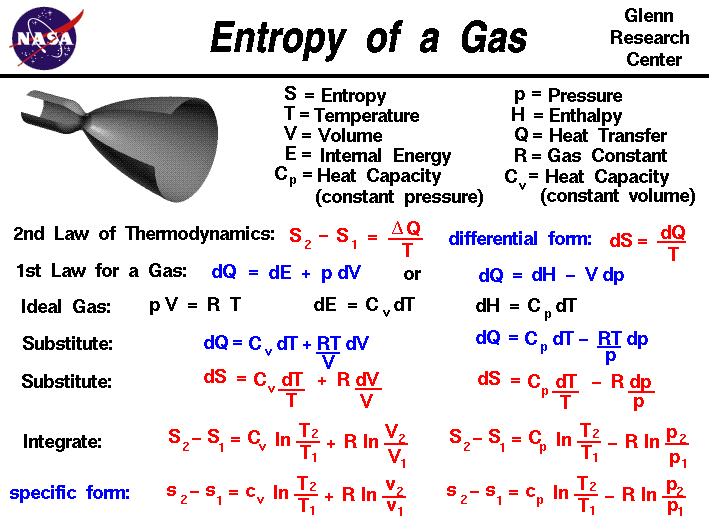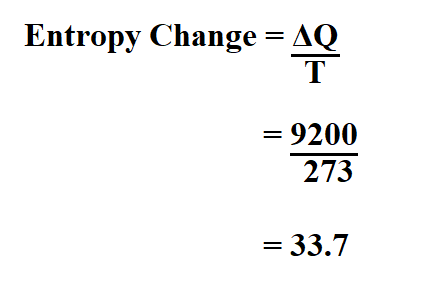PPT - Entropy Changes in the Surroundings Now that we have seen how to calculate D S system for reversible and irreversible PowerPoint Presentation - ID:28591

SOLVED: Calculate the entropy change of the universe (J/mol-K) when the entropy change of the system is 59.4 J/mol-K and the surroundings absorb 33.71 kJ of heat from the system at 77.74 °

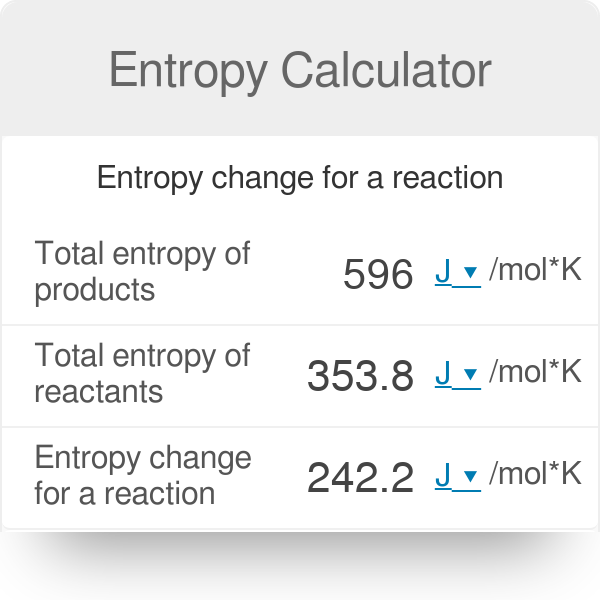
Calculate the entropy change in surroundings when 1.00 mol of { H }_{ 2 }O (l) is formed under standard conditions 298k, Given triangle ,{ H }^{ o }quad =quad -286kJquad { mol }^{ -1 }

Can you calculate the entropy change when one mole of an ideal gas expands to double its original volume? - Quora
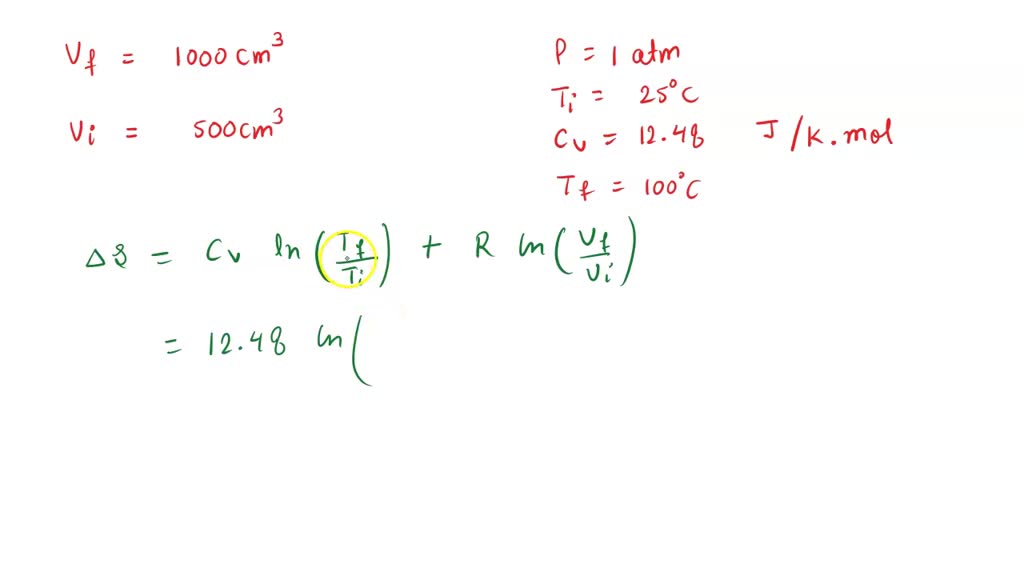
SOLVED: Calculate the entropy change when argon at 25 °C 1.00 atm in a container of volume 500 cm3 was allowed to expand to 1000 cm3 and is simultaneously heated to 100
Calculate the entropy change in the system. and in the surroundings and the total entropy change in the universe when during - Sarthaks eConnect | Largest Online Education Community

22. Calculate the entropy change when 2.8 g of N, gas expands isothermal e entopy change when 2.8 g of N, gas expands isothermally and reversibly from an initial volume of 1

calculate the entropy change when 1 mole of an ideal gas is allowed to expand isothermally at 315k and pressure 5atm to 2.5atm - EduRev Class 11 Question