Enthalpy of combustion of alkanes graph vales versus carbon number molecular mass Complete & incomplete combustion of alkanes environmental pollution problems advanced A level organic chemistry revision notes
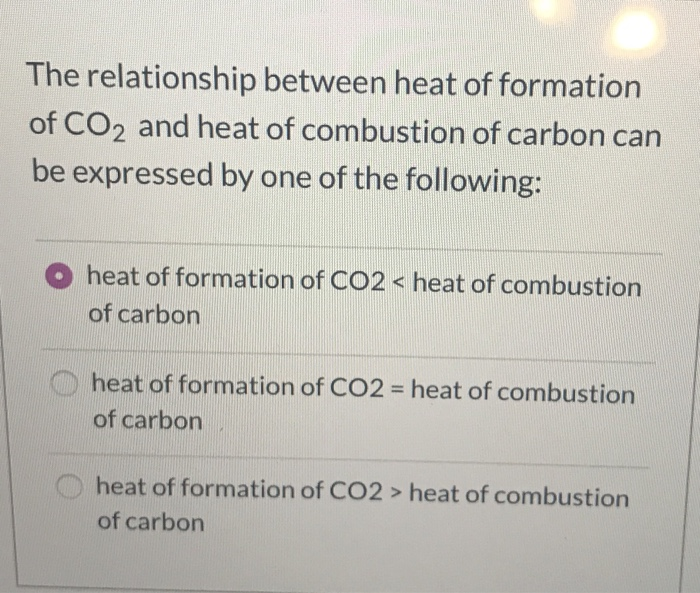
The heat of combustion of carbon to CO2 is 395.5kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is

8. The heat of combustion of carbon to CO. -393.5 kJ/ mol. The heat released upon formation d 35.2 g of Co, from carbon and oxygen gas is [Re-AIPMT-2015 (1) -630 kJ (
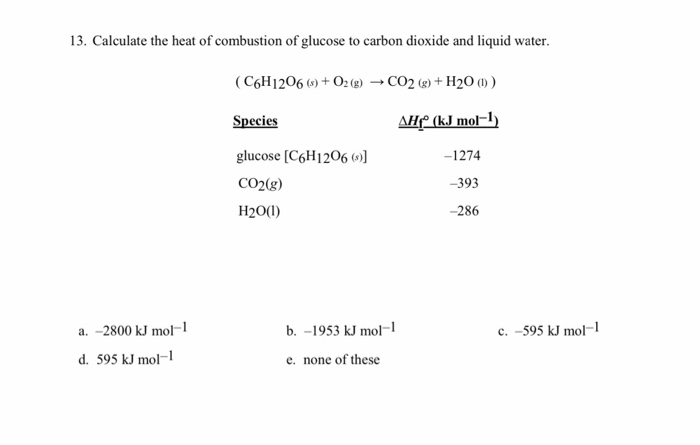
The combustion enthalpies of carbon, hydrogen, and methane are 395.5 kJ mol^ 1, 284.8 kJ mol^ 1 and 890.4 kJ mol^ 1 respectively at 25^0C. The value of s†an dard formation enthalpies
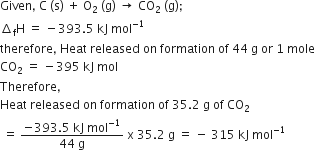
The heat of combustion of carbon to CO2 is -395.5 kJ/mol. The heat released upon the formation of 35.2 g CO2 from carbon and oxygen gas is - Zigya

enthalpy of combustion of carbon to co2 is -393 5 kilo joule per mole calculate the heat produced upon the - Chemistry - Thermodynamics - 13291531 | Meritnation.com
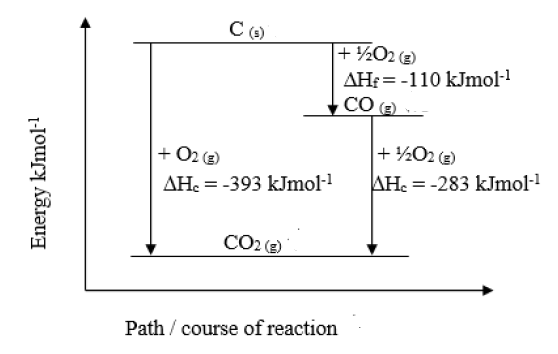
Given that: Enthalpy of combustion of Carbon (graphite) = -393kJmol^-1 Enthalpy of combustion of - Tutorke

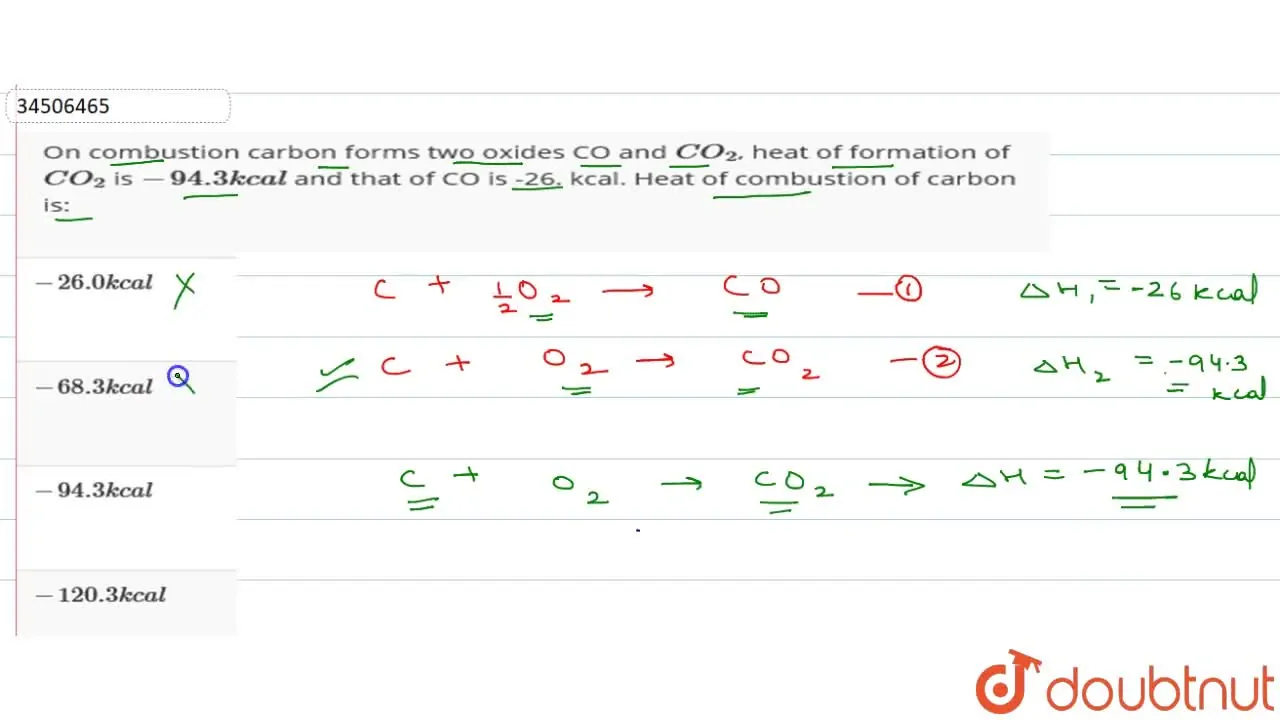
On combustion carbon forms two oxides CO and {CO}_{2}, heat of formation of {CO}_{2} is -94.3 kcal and that of CO is -26.0 kcal. Heat of combustion of carbon is:-26.0 kcal-68.3 kcal-120.3
The heat of combustion of carbon to CO2 is 393.5Kj/mol. The heat rrleased upon formtion of 35.2 g of CO2 from carbn and oxygem gad is
62.The Enthalpies of combustion of carbon and carbon monoxide are 390 kJ and 278kJ respectively. The enthalpy of formation of carbon monooxide is? a) 669 kJ b) 112 kJ c) 112 kJ d) 668 kJ